27 Dec Pilot Study on Safety & Acceptability of IntegriMedical® Needle-Free vs. Needle Injection Using Saline
Almas Pathan,1 Kavitha Shetty Narasimha,2 Ankur Naik,2 Arati Ranade1
1Jehangir Clinical Development Centre, Pune, Maharashtra, India; 2IntegriMedical Private Limited, Pune, Maharashtra, India
Correspondence: Almas Pathan, Jehangir Clinical Development Centre, Jehangir Hospital Premises 32, Sassoon Road, Pune, Maharashtra, 411 001, India, Email almas.jikare@rediffmail.com
Purpose: The study aimed to assess the safety, tolerability, and acceptability of the IntegriMedical® Needle Free Injection System (IM-NFIS) compared to conventional hypodermic needle injections (CHN) in healthy adult subjects across multiple injection sites.
Patients and Methods: Thirty healthy male subjects aged 18– 45 years were enrolled in this open-label study. Each subject received both NF and CHN injections at five different sites (forearm, abdomen, thigh, buttocks, and arm). In the study, participants in the forearm cohort received 0.1 mL of saline, whereas, for all other injection sites, 0.5 mL of saline was administered. Both needle-free and hypodermic needle injections were used at the designated sites, with a 5- to 10-minute interval between each injection. Since no active drug was used, saline served as a placebo in both methods. Safety assessments included local and systemic reactions, pain scores using the Visual Analog Scale (VAS), and acceptability questionnaires. The study adhered to ethical guidelines and was approved by the Institutional Ethics Committee.
Results: NF injections demonstrated significantly lower pain scores compared to CHN injections (mean VAS score 0.23 vs 1.07, p < 0.01). Local site reactions were minimal and similar between NF and CHN injections, with no significant differences observed at 20– 30 minutes post-injection. Systemic reactions were absent in both groups throughout the study period. The NF injection system was highly acceptable, with a majority of subjects reporting reduced anxiety and pain compared to CHN injections (p < 0.01).
Conclusion: IM-NFIS proved to be safe, well tolerated, and highly acceptable for delivering pharmaceuticals compared to conventional needle and syringe injections. This needle-free technology offers potential advantages in improving patient compliance and reducing injection-related anxieties, suggesting its promising role in future medical practices, including pediatric vaccinations and frequent medication administration.
Keywords: medical device, acceptability questionnaire, pain, VAS score, US-FDA toxicity scale, comparative study
Introduction
Injections with hypodermic needles are a common procedure in modern medicine. Ideally, a solution of a drug is forced under piston stress straight into the bloodstream or exact tissue. This necessitates skin perforation using a needle, which is associated with trauma and pain. To overcome these drawbacks, other alternative methods have been investigated like jet injections, dermabrasion, thermal ablation, laser, tape stripping, etc.1 Reduction of the pain may lead to improved patient satisfaction and compliance, as well as reduced anxiety in populations of patients who require frequent vaccinations or ongoing injections to treat their medical conditions.2
Needle-free injection systems have emerged as an innovative solution, delivering medications without traditional needles. The concept originated with Marshall Lockhart in 1936 and was advanced in the 1940s by Higson et al, who developed high-pressure devices that use fine liquid jets to penetrate the skin.3 Needle-free jet injectors gained popularity with the introduction of multi-use nozzle jet injectors (MUNJIs), which were utilized by the US military between 1947 and 1965 due to their ability to administer vaccinations at a high rate.4 Multi-use nozzle jet injectors (MUNJIs) were widely used for mass immunization campaigns until the 1980s. However, in 1985, a hepatitis B outbreak was linked to MUNJIs, likely due to contamination from body fluids. To address this, single-use nozzle devices, known as disposable cartridge jet injectors (DCJIs), were developed. These use a disposable cartridge and nozzle to prevent blood from splashing back onto the device, reducing the risk of contamination.5 Since the early 2000s, technological advancements have enhanced needle-free injection systems (NFIS), making them more comfortable, accurate, and user-friendly.6 Recently, there even has been a significant shift in technology, leading to increased interest in the use of needle-free injection methods.7 Currently, several notable devices, including PharmaJet Stratis, Biojector 2000, and Zetajet, are commonly used for administering vaccines and managing pain. Today, even systems like microarray patches serve as alternative to conventional hypodermic needle.8 The working principle of needle free injection works on different technologies including spring system, gas propelled system, etc.9
All these systems offer numerous advantages, including the elimination of puncture-related hazards, minimized bleeding and bruising, and faster, more reproducible drug delivery with enhanced bioavailability. They also improve drug stability, reduce needle phobia particularly benefiting vaccine delivery and the development of drug proteins. However, these systems are complex and costly, requiring trained personnel and are not suitable for intravenous use.3 Nonetheless, advantages of the needle-free injection system over power its limitations.
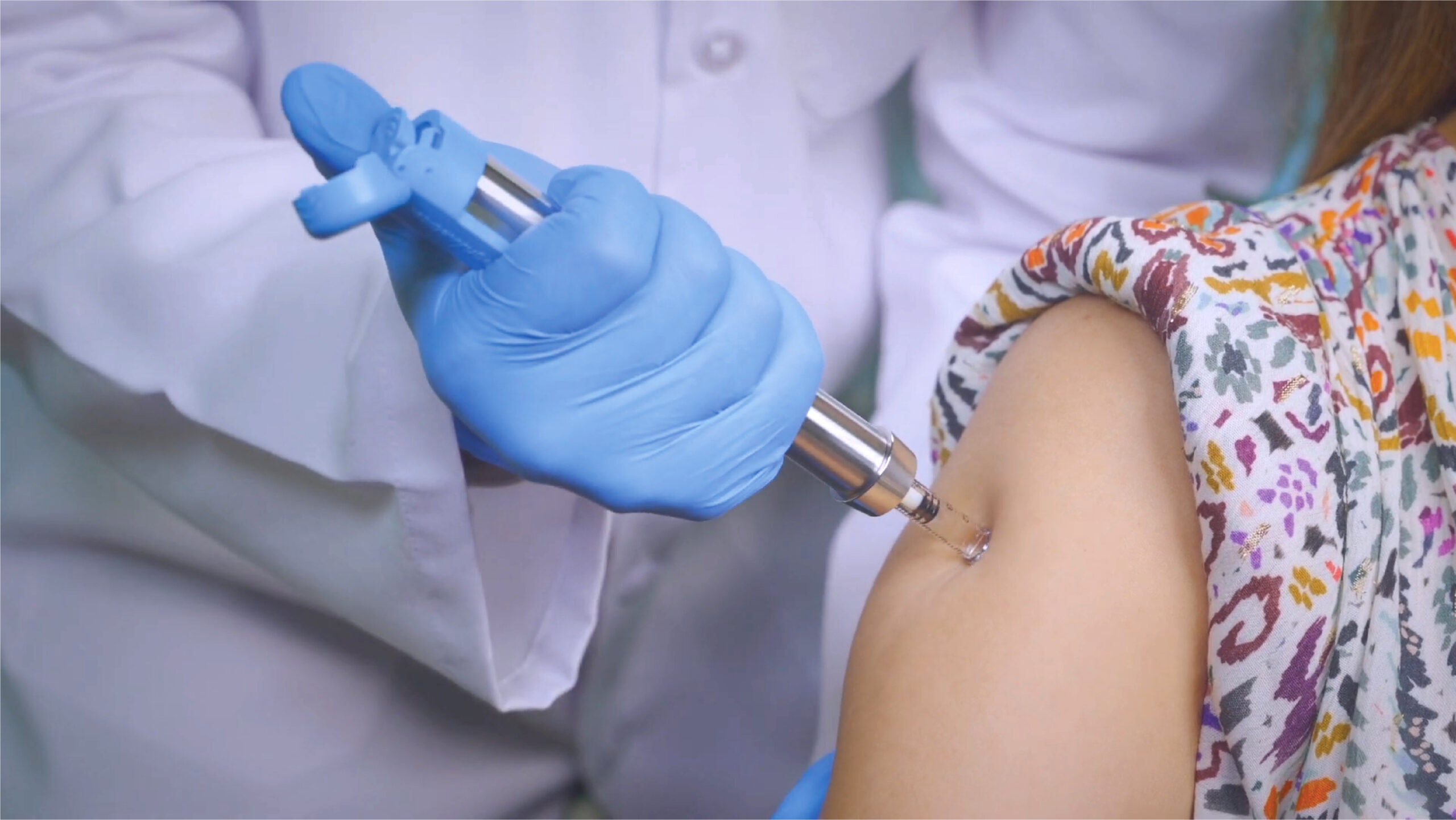
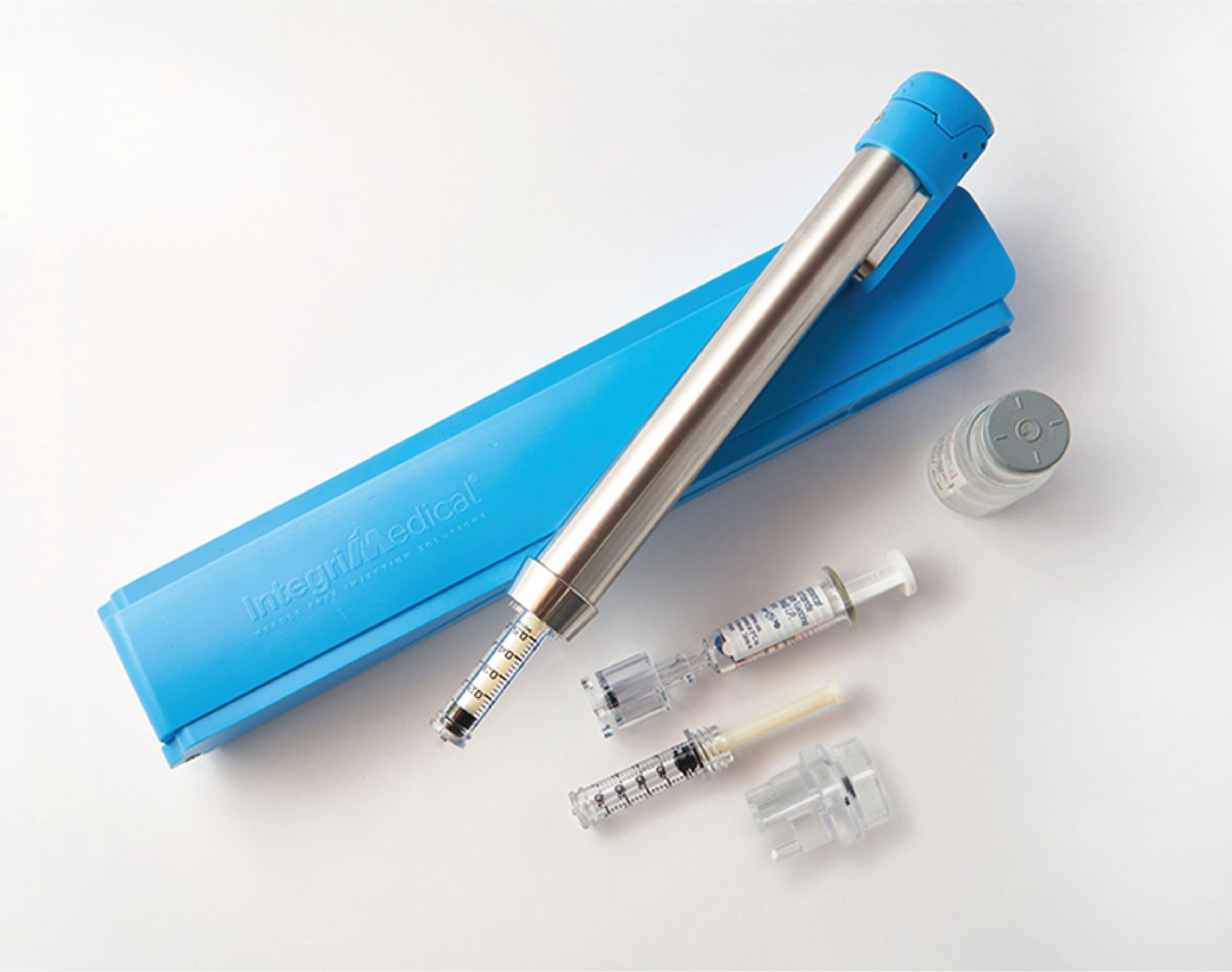
IntegriMedical® Needle Free Injection System (IM-NFIS) is intended to deliver drugs and biologics through a needle-free injection device. IM-NFIS (Figure 1) works on needle-free injection technology using the principle of generation of a high-pressure jet, which pierces the skin when it comes in intimate contact with the skin and thus delivering the liquid it contains into the tissues in and under the skin. In the NFIS, potential energy is stored in the loaded spring, which when suddenly released by trigger pushes the plunger and attached piston into the cylinder and creates a pressure surge in contained liquid drug or biologic which then escapes as a high-velocity jet stream through micro-orifice of the cartridge. Typical doses can range from 0.1 mL to 0.5 mL and are delivered to various injection depths such as Intradermal (ID), Subcutaneous (SC), and Intramuscular (IM) depending on the volume of the drug or biologic loaded in the Cartridge. The ID injection depth occurs just under the epidermal layer of skin and is usually for a smaller dose of 0.1mL. The SC and IM injection depths occur under the ID layer and are usually intended for larger doses.
The present study was the first in human assessment of IM-NFIS to compare safety, tolerability and acceptability of needle-free injection system in healthy subjects with conventional hypodermic needle-based system at 5 different sites of administration (forearm, abdomen, thigh, buttocks and arm).
Material and Methods
Participants and Ethics
Subjects were enrolled in the study only after providing signed informed consent. Eligible participants included males and females aged 18 to 45 who were in good general health, had no significant medical history and showed no notable findings during physical examinations. Participants also had no history of liver, kidney, cardiovascular, or neurological disorders in the past three months, and female participants were non lactating and tested negative for pregnancy on the day of enrollment.
Individuals who recently used immunosuppressive or cytotoxic medications, received blood products within the last 16 weeks, or had a history of bleeding disorders were excluded from the study. The study was conducted at Jehangir Clinical Development Centre in Pune, India. The study protocol (IM/NFIS/01) was approved by the Institutional Ethics Committee (ECR/352/lnst/MH/2013/RR-19) and the study was registered with the Clinical Trial Registry of India (CTRI/2021/01/030587). The study adhered to ethical guidelines outlined in the “Ethical Principles for Medical Research Involving Human Subjects”, as specified in the Helsinki Declaration.10
Study Design
This was a 5-day open label study conducted in 30 subjects divided into 5 cohorts with 6 subjects in each cohort. Subjects were randomized sequentially on the basis of their enrolment in the study into the following cohorts- The forearms (Cohort 1), abdomen (Cohort 2), thighs (Cohort 3), buttocks (Cohort 4), and arms (Cohort 5). Each subject acted as both test (Saline delivery through Needle free injection) and control (Saline delivery through Hypodermic needle). Every site was divided into designated areas for test and control treatments. The right side of each site received saline via the needle-free injection system (NFIS), while the left side was treated with a hypodermic needle (Table 1).
 |
Table 1 Cohorts and Areas for Receiving the Product |
The study consisted of five visits. Visit 1 (Baseline/Screening/Day 0) involved an initial evaluation of physical examination, vital signs assessment, clinical laboratory tests (including hematology and serum creatinine), and a chest X-ray to assess the eligibility of the subject. On Visit 2 (Day 1/Enrolment), participants in Cohorts 2 to 5 received 0.5 mL of saline placebo administered by the principal investigator using both a IntegriMedical® needle-free injection and a conventional hypodermic needle at the designated site. Participants in Cohort 1 received 0.1 mL of saline placebo administered in the same way, with a 5- to 10-minute interval between injections.11 The principal investigator assessed the injection sites at 2 minutes and again between 20–30 minutes after administration. Subjects were evaluated for site reactions and systemic reactions using US-FDA toxicity scale guidance,12 Pain levels were assessed using the Visual Analog Scale (VAS),13 with scores categorized as None (0), Mild (1–3), Moderate (4–6), and Severe (7–10) and acceptability using inhouse questionnaire separately after each injection.14 Also, participants were trained to monitor local site reactions and reported both local and systemic reactions over the phone, sending photos of injection sites to the investigators. Visits 3 (Day 2) and visit 4 (Day 3) was the telephonic visits, follow-up assessments via telephone focused on AEs/SAEs using toxicity scale provided by FDA guidance. Those participants who experienced site reactions were called in for further evaluation. A final visit 5 follow-up on Day 4 similarly assessed AEs/SAEs, marking the end of the study (EOS).
Sample Size
A total of 30 subjects were recruited in this study based on the inclusion/exclusion criteria. According to the FDA guidance for pilot or feasibility study of medical devices, endpoints and sample size need not be statistically driven and generally can involve around 10–40 subjects. Studies on similar devices also show use of small population for establishing safety of the device.15,16 This justifies the selection of 30 participants in this study.
Data Analysis
Statistical analysis was performed using the SPSS Version 20 software. All available data was used in the analyses. All comparative analysis was based on two-tailed tests with a significance level of α = 0.05. The study endpoints were tested for statistical significance using paired t test/chi-square test as appropriate.
Results
The demographic and patient characteristics show (Table 2) that all the subjects enrolled were male with mean age of 26.2 years (median 22.5 years; range 18 to 43 years). Highest percentage of subjects (43.3%) were in the age range of 18–20 years, with 30.0% in the range 21–30 years and 26.7% above 31 years. Mean weight was noted as 61.6 kg (SD 11.6 kg) with minimum of 44 kg and maximum of 89 kg. Mean height was recorded as 169.2 cm (SD 7.6 cm) with minimum 146 cm and maximum 181 cm. All except one subject were non-smokers and non-alcoholics. Subjects’ healthy status was ensured through the measurement of vitals, physical examination and collection of medical history. All subjects were reported to have normal clinical relevance on above parameters. Figure 2 highlights the subject disposition pattern.
 |
Table 2 Demographic Characteristics of the 30 Subjects at Baseline |
 |
Figure 2 CONSORT Flow Diagram of Study Participants. This flowchart summarizes participant progression through the study stages, including enrollment, allocation, follow-up, and analysis. |
Safety Assessments Through Local Site Reactions
Data on local site reactions collected at 2 min and at 20–30 min following NF injection and CHN injection is summarized in the Table 3. Post 2 min, one subject (receiving NF injection in Arm) and three subjects post CHN injection (two in Arm and one in Abdomen) reported Grade 1 pain (does not interfere with activity). Grade 1 tenderness (mild discomfort to touch) was reported by two each, NF injection (both Forearm) and CHN injection (one Forearm and one Abdomen) subjects. None reported erythema/redness or induration. At 20–30 min post injections no local site reactions was reported for both injection methods. Further, it is worth noting that when compared to conventional needle the difference obtained in site reactions has not reached a statistical significance level.
 |
Table 3 Summary of Local Reactions Post Injections (2 min and 20–30 min) |
Data for all 30 study subjects who received both NF and CHN injections and collected at 24, 48, and 72 hours post injection (Table 4) revealed that none of the subjects reported any kind of complaints on all the three instances.
 |
Table 4 Summary of Local Reactions Post Injections (24, 48 and 72 hours) |
Safety Assessments Through Systemic Reactions
Systemic examination (nausea, diarrhea, headache, fatigue, and myalgia) carried out for after NF and CHN injections administration at 2 min and 20–30 minutes (Table 5) demonstrates that none of the subjects in both injection types reported any adverse events. This conclusion was valid across both groups at 20–30-minute post injections also.
 |
Table 5 Systemic Examination Post Injections (2 min and 20–30 min) |
Further, systemic examinations carried out for all subjects post 24, 48, and 72 hours (Table 6) after injections also revealed that none of the subjects reported any adverse events.
 |
Table 6 Systemic Examination Post Injections (24, 48 and 72 hours) |
Pain Assessment Using VAS Score
Pain score when assessed within 2 min following the NF injection and CHN injection showed 76.7% of the subjects reported no pain post NF injection compared with 30.0% in the CHN injection recipients (Table 7). The percentage of those who did not experience pain post NF injection was significantly higher as compared with CHN injection group (P < 0.01; chi-square test). Mean pain score for the NF injection was 0.23 and for CHN injection it was reported as 1.07. The lower pain score post NF injection as compared with CHN injection was statistically significant (P < 0.01; paired-t test for comparison of 2 means). Hence, tolerability of NFIS was proven through this study.
 |
Table 7 VAS Pain Score Assessment Following NF and CHN Injections |
Acceptability Questionnaire
Acceptability questionnaire was administered to the study subjects post administration of NF injection and CHN injection. Responses given by the subjects separately for the two injections are tabulated in Table 8. The NF injection was generally acceptable with many questions responded as “not at all” by all subjects. Significantly higher percentage (90%) responded that they did not feel anxious about receiving the NF injection as compared with 43.3% with the CHN injection (P <0.01; 2 × 2 chi-square test with continuity correction). All the subjects (100%) were not bothered by pain during the NF injection as compared with 43.3% in the CHN injection (P < 0.01; 2 × 2 chi-square test with continuity correction).
 |
Table 8 Acceptability Responses Given by Subjects’ Post NF and CHN Injections |
Discussion
All 30 subjects in this study received both injections. At the time of screening, vital signs of the study subjects had “normal” body temperature, heart rate, respiratory rate, and blood pressure. All subjects in both the groups were “normal” with respect to general appearance, head, ENT, eyes, skin, neck, abdomen, cardiovascular, respiratory, musculoskeletal, neurological, and lymphatic systems. None of the study subjects reported any past / current medical history. These findings ensured the healthy status of the subjects during the recruitment.
Subjects were first administered saline with needle free injection (NF Injection) system followed by conventional hypodermic needle injection (CHN Injection). NF injection was given in the right side and the CHN injection on the left side. Five injection sites were used – forearm, abdomen, buttock, thigh, and arm with six subjects in each group. Post NF injection, VAS pain score was recorded within 1 min for all subjects. In the case of CHN injection, VAS pain score was recorded within 2 minutes for 28 subjects and for remaining 2 subjects the measurement was completed in 3 minutes. FDA toxicity assessments were done within 02 min for all subjects post NF injection and CHN injection. Toxicity assessments were repeated post 20–30 min of each injection. These assessments did not highlight any safety concern following the administration of injections during the entire planned follow-up period. VAS pain assessment scores demonstrated that the NF injection induced (statistically) significantly lower pain scores as compared with CHN injection. NF injection was well tolerated as that of the convention injection (CHN injection). Hence, tolerability of NFIS was proven through this study. Only one subject complained of pain post NF injection after 2 min, three following CHN injection. Tenderness was reported by two subjects for both injection types after 2 min. No other local reactions were noted. Our findings were similar to the results of a clinical study that assessed the safety, tolerability and pharmacokinetics of enfuvirtide administered by a needle-free injection system compared with subcutaneous injection.17
Vitals remained stable post injection and systemic examination did not highlight any complaints. Questionnaire responses suggest that IM-NFIS system was well accepted. These results can be correlated with the results of a PRCISE-II clinical study18 that assessed safety and subject preference for an innovative needle-free injection system. Overall, NF injection had a significantly higher satisfaction percentage compared with the CHN injection administration. None of the subjects reported any specific adverse events. The Needle Free Injection System (NFIS) provided easier, less painful, and more acceptable delivery technology for a layperson making it more user-friendly than traditional needles.
The study’s findings suggest that this study have several important implications. The IntegriMedical® Needle Free Injection System (IM-NFIS) could enhance patient compliance and satisfaction by reducing pain and anxiety compared to conventional hypodermic needles, making it particularly beneficial for populations with needle phobia, such as children or individuals requiring frequent injections, thereby potentially improving vaccination rates and adherence to treatment regimens. Additionally, NFIs help prevent needle-stick injuries and the risk of needle reuse, minimizing infection risks in children.19,20
Limitations
The small sample size of thirty healthy male subjects restricts the generalizability of the results to a wider population. Additionally, the use of saline as a placebo may not accurately represent responses to actual medications. These factors underscore the need for further research to gain deeper insights into the safety and effectiveness of the IntegriMedical® Needle Free Injection System.
Conclusion
In summary, IM-NFIS demonstrated safety, tolerability and acceptability compared to a conventional needle and syringe injection. The advancement of needle-free injection technology has potential positive implications especially for patients who require frequent injections of medications for a variety of conditions. The use of this technology can significantly benefit children by minimizing discomfort, reducing anxiety associated with needles, and improving overall compliance with vaccination schedules. Further studies are needed to evaluate the effectiveness of the device in successful delivery of pharmaceuticals or biologicals.
Data Sharing Statement
The data are not publicly available due to their containing information that could compromise the privacy of patients. All data supporting this study will be provided by the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was conducted according to the guidelines of the Declaration of Helsinki. This study protocol was approved by the Institutional Ethics Committee of Jehangir Clinical Development Centre. The informed consent was taken from the subject.
Acknowledgments
Authors are thankful to Jehangir Clinical Development Centre Pvt. Ltd., Pune for designing the clinical study, conduct of the study and statistical analysis of the data.
Disclosure
Mr Ankur Naik reports a patent US20230414876A1 issued to INTEGRIMEDICAL LLC, a patent US11752269B2 issued to INTEGRIMEDICAL LLC. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Mishra DK, Pandey V, Maheshwari Piyush Ghode R, Tekade KR, Basic Fundamentals of Drug Delivery Advances in Pharmaceutical Product Development and Research. Chapter 15 – Cutaneous and Transdermal Drug Delivery. Techniques Delivery Syst. 2019;2019:595–650.
2. Barolet D, Benohanian A. Current trends in needle-free jet injection: an update. Clin Cosmet Invest Dermatol. 2018;11:231–238. doi:10.2147/CCID.S162724
3. Kale TR, Momin M. Needle free injection technology-An overview. Innovations in Pharmacy. 2014;5(1):1.
4. Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5(7):543–548. doi:10.1038/nrd2076
5. du Châtelet IP, Lang J, Schlumberger M, Vidor E, Soula G, Genet A, Imule® Investigators Group. Clinical immunogenicity and tolerance studies of liquid vaccines delivered by jet-injector and a new single-use cartridge (Imule®): comparison with standard syringe injection. Vaccine. 1997;15(4):449–458. doi:10.1016/S0264-410X(96)00173-9
6. Schoppink J, Rivas DF. Jet injectors: perspectives for small volume delivery with lasers. Adv. Drug Delivery Rev. 2022;182:114109. doi:10.1016/j.addr.2021.114109
7. Mohizin A, Kim JK. Current engineering and clinical aspects of needle-free injectors: a review. J Mech Sci Technol. 2018;32(12):5737–5747. doi:10.1007/s12206-018-1121-9
8. Berger MN, Mowbray ES, Farag MW, et al. Immunogenicity, safety, usability and acceptability of microarray patches for vaccination: a systematic review and meta-analysis. BMJ Global Health. 2023;8(10):e012247. doi:10.1136/bmjgh-2023-012247
9. Ravi AD, Sadhna D, Nagpaal D, Chawla L. Needle free injection technology: a complete insight. Int J Pharm Investig. 2015;5(4):192–199. doi:10.4103/2230-973X.167662
10. WMA. WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Participants. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki/#:~:text=All%20medical%20research%20involving%20human,by%20the%20condition%20under%20investigation. Accessed 30, October 2024.
11. Kojic N, Goyal P, Lou CH, Corwin MJ. An Innovative Needle-free Injection System: comparison to 1 mL Standard Subcutaneous Injection. AAPS PharmSciTech. 2017;18(8):2965–2970. doi:10.1208/s12249-017-0779-0
12. FDA Guidance for Industry. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. CBER; 2007.
13. Dias C, Abosaleem B, Crispino C, Gao B, Shaywitz A. Tolerability of High-Volume Subcutaneous Injections of a Viscous Placebo Buffer: a Randomized, Crossover Study in Healthy Subjects. AAPS Pharm Sci Tech. 2015;16(6):1500. doi:10.1208/s12249-015-0324-y
14. Chevat C, Viala-Danten M, Dias-Barbosa C, Nguyen VH. Development and psychometric validation of a self-administered questionnaire assessing the acceptance of influenza vaccination: the Vaccinees’ Perception of Injection (Vapi) questionnaire. Health Qual Life Outcomes. 2009;7(1):21. doi:10.1186/1477-7525-7-21
15. https://www.fda.gov/media/87603/download;.
16. Berteau C, Filipe-Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Dev. 2015;8:473–484. doi:10.2147/MDER.S91019
17. Gottlieb M, Thommes JA. Safety, tolerability and pharmacokinetics of enfuvirtide administered by a needle-free injection system compared with subcutaneous injection. Antiviral Ther. 2008;13(5):723–727. doi:10.1177/135965350801300512
18. Kelley EL, Smith RH, Corcoran G, Nygren S, Jacoski MV, Fernandes A. Advances in subcutaneous injections: PRECISE II: a study of safety and subject preference for an innovative needle-free injection system. Drug Deliv. 2021;28(1):1915–1922. doi:10.1080/10717544.2021.1976309
19. Abd Ellatif EM. Comparison Between needle-less injection system and Conventional injection Technique to Perform anesthesia In Children: a Randomized Clinical Trial. Egypt Dent J. 2018;64(3):1981–1985. doi:10.21608/edj.2018.76697
20. Kelly K, Loskutov A, Zehrung D, et al. Preventing contamination between injections with multiple-use nozzle needle-free injectors: a safety trial. Vaccine. 2008;26(10):1344–1352. doi:10.1016/j.vaccine.2007.12.041